| | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Oxamide[1] | |||
| Systematic IUPAC name Ethanediamide | |||
| Other names Oxalamide Oxamimidic acid Diaminoglyoxal Oxalic acid diamide 1-Carbamoyl-formimidic acid | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.767 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
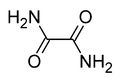
Chemical formula | C2H4N2O2 | ||
| Molar mass | 88.0654 g/mol | ||
| Appearance | White powder | ||
| Density | 1.667 g/cm3 | ||
| Soluble | |||
| Solubility | ethanol | ||
| -39.0·10−6 cm3/mol | |||
| Hazards | |||
EU classification (DSD) (outdated) | Mild Irritant (6.1) | ||
| R-phrases (outdated) | R36 | ||
| S-phrases (outdated) | S25 | ||
| Flash point | > 300 °C (572 °F; 573 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Oxamide is the organic compound with the formula (CONH2)2. This white crystalline solid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid.
Preparation
Oxamide is produced from hydrogen cyanide, which is oxidized to cyanogen, which is then hydrolyzed.[2]
It can also be prepared from formamide by glow-discharge electrolysis.[3]
Application
The main application is as a substitute for urea in fertilizers. Oxamide hydrolyzes (releases ammonia) very slowly, which is sometimes preferred vs the quick release by urea.
It is used as a stabilizer for nitrocellulose preparations. It also finds use in APCP rocket motors as a high performance burn rate suppressant. The use of oxamide in concentrations of 1-3 wt% has shown to slow the linear burn rate while having minimal impact on propellant specific impulse.
N, N'-substituted oxamides are supporting ligands for the copper-catalyzed amination and amidation of aryl halides in (Ullmann-Goldberg reaction), including relatively unreactive aryl chloride substrates.[4]
Reactions
It dehydrates above 350 °C releasing cyanogen. Oxamide derivatives form self-assembled monolayers consisting of a hydrogen bonded network.[5]
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 841. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Riemenschneider, Wilhelm; Tanifuji, Minoru (2002). "Oxalic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_247..
- ^ Brown, E. H.; Wilhide, W. D.; Elmore, K. L. (1962). "A New Method for the Preparation of Oxamide". The Journal of Organic Chemistry. 27 (10): 3698. doi:10.1021/jo01057a516.
- ^ Zhou, Wei; Fan, Mengyang; Yin, Junli; Jiang, Yongwen; Ma, Dawei (2015-09-23). "CuI/Oxalic Diamide Catalyzed Coupling Reaction of (Hetero)Aryl Chlorides and Amines". Journal of the American Chemical Society. 137 (37): 11942–11945. doi:10.1021/jacs.5b08411. ISSN 0002-7863. PMID 26352639.
- ^ Nguyen T.L., Fowler F.W., Lauher J.W., "Commensurate and incommensurate hydrogen bonds. An exercise in crystal engineering." Journal of the American Chemical Society, 123(44), pp. 11057-64, 2001. doi:10.1021/ja016635v