 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Methyl-1,2-thiazol-3(2H)-one | |
| Other names 2-Methylisothiazol-3(2H)-one 2-Methyl-4-isothiazolin-3-one | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | MIT |
| 606203 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.399 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C4H5NOS |
| Molar mass | 115.1 g/mol |
| Appearance | white solid |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements | H301, H311, H314, H317, H318, H330, H400, H410 |
GHS precautionary statements | P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P333+313, P361, P363, P391 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
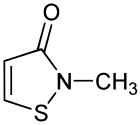
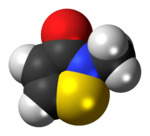
Methylisothiazolinone, MIT, or MI, is the organic compound with the formula S(CH)2C(O)NCH3. It is a white solid. Isothiazolinones, a class of heterocycles, are used as biocides in numerous personal care products and other industrial applications. MIT and related compounds have attracted much attention for their allergenic properties, e.g. contact dermatitis.[1]
It is prepared by cyclization of cis-N-methyl-3-thiocyanoacrylamide:[2]
- NCSCH=CHC(O)NHCH3 → SCH=CHC(O)NCH3 + HCN
Safety
MIT is allergenic and cytotoxic, and this has led to some concern over its use.[3] It has attracted concern in the U.S. and in Europe.[4][5] Methylisothiazolinone may cause respiratory irritation, skin sensitivities (including dermatitis), skin burns and eye damage according to peer-reviewed research and data from the European Chemicals Agency (ECHA) reported as part of the Environmental Working Group’s ‘Guide to Healthy Cleaning’. The American Contact Dermatitis Society named Methylisothiazolinone “allergen of the year” in 2013.[6]
References
- ^ Silva, Vânia; Silva, Cátia; Soares, Pedro; Garrido, E. Manuela; Borges, Fernanda; Garrido, Jorge (2020). "Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles". Molecules. 25 (4): 991. doi:10.3390/molecules25040991. PMC 7070760. PMID 32102175.
- ^ Crow, W. D.; Leonard, Nelson J. (1965). "3-Isothiazolone-cis-3-Thiocyanoacrylamide Equilibria1,2". The Journal of Organic Chemistry. 30 (8): 2660–2665. doi:10.1021/jo01019a037.
- ^ Castanedo-Tardana, M. P.; Zug, K. A. (2013). "Methylisothiazolinone". Dermatitis : Contact, Atopic, Occupational, Drug. 24 (1): 2–6. doi:10.1097/DER.0b013e31827edc73. PMID 23340392. S2CID 220573338.
- ^ MCI/MI/BIT Call To Action!
- ^ European Scientific Committee on Cosmetic Products and Non-food Products Intended for Consumers (SCCNFP), adopted 2004
- ^ Why Methylisothiazolinone-Free Cleaning Products Matter
External links
- 2014 EPA Re-registration Review Docket with Public Commentary
- Reregistration Eligibility Decision of MIT by US EPA
- Record in the Household Products Database of NLM
- Material Safety Data Sheet for product containing 0.1-1% MIT
- Commission Scientific Committee on Consumer Safety Opinion on Methylisothiazolinone (P94) Submission II (Sensitization only) SCCS/1521/13
- Methylisothiazolinone at the National Library of Medicine
- 2014 EPA Re-registration Review Docket with Public Commentary
- Photo album of self reported reactions to methylisothiazolinone