 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.149 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 1617 |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
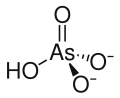
Chemical formula | AsHO4Pb |
| Molar mass | 347.1 g·mol−1 |
| Appearance | white solid |
| Density | 6.07 g/cm3 |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements | H301, H331, H350, H360Df, H373, H400, H410 |
GHS precautionary statements | P201, P202, P260, P261, P264, P270, P271, P273, P281, P301+310, P304+340, P308+313, P311, P314, P321, P330, P391, P403+233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lead hydrogen arsenate, also called lead arsenate, acid lead arsenate or LA, chemical formula PbHAsO4, is an inorganic insecticide used primarily against the potato beetle. Lead arsenate was the most extensively used arsenical insecticide.[1] Two principal formulations of lead arsenate were marketed: basic lead arsenate (Pb5OH(AsO4)3, CASN: 1327-31-7) and acid lead arsenate (PbHAsO4.[1]
Production and structure
It is usually produced using the following reaction, which leads to formation of the desired product as a solid precipitate:
- Pb(NO3)2 + H3AsO4 → PbHAsO4 +2 HNO3
It has the same structure as the hydrogen phosphate PbHPO4. Like lead sulfate PbSO4, these salts are poorly soluble.[2]
Uses
As an insecticide, it was introduced in 1898 used against the gypsy moth in Massachusetts. It represented a less soluble and less toxic alternative to then-used Paris Green, which is about 10x more toxic.[3] It also adhered better to the surface of the plants, further enhancing and prolonging its insecticidal effect.
Lead arsenate was widely used in Australia, Canada, New Zealand, US, England, France, North Africa, and many other areas, principally against the codling moth and snow-white linden moth.[4] It was used mainly on apples, but also on other fruit trees, garden crops, turfgrasses, and against mosquitoes. In combination with ammonium sulfate, it was used in southern California as a winter treatment on lawns to kill crab grass seed.
The search for a substitute was commenced in 1919, when it was found that its residues remain in the products despite washing their surfaces. Alternatives were found to be less effective or more toxic to plants and animals, until 1947 when DDT was found. US EPA banned use of lead arsenate on food crops in 1988.[5][6]
Safety
LD50 is 1050 mg/kg (rat, oral).[3]
Morel mushrooms growing in old apple orchards that had been treated with lead arsenate may accumulate levels of toxic lead and arsenic that are unhealthy for human consumption.[7]
Lead arsenate was used as an insecticide in deciduous fruit trees until around 1947 in Washington. Peryea et al. studied the distribution of Pb and As in these soils, concluding that these levels were above maximum tolerance levels. This indicates that these levels could be of environmental concern and potentially could be contaminating the groundwater in the area. [8]
See also
References
- ^ a b Peryea F.J. 1998. Historical use of lead arsenate insecticides, resulting in soil contamination and implications for soil remediation. Proceedings, 16th World Congress of Soil Science, Montpellier, France. 20-26. Aug. Available online: http://soils.tfrec.wsu.edu/leadhistory.htm Archived 2008-12-07 at the Wayback Machine
- ^ Wilson, C. C.; Cox, P. J.; Stewart, N. S. (1991). "Structure and disorder in schultenite, lead hydrogen arsenate". Journal of Crystallographic and Spectroscopic Research. 21 (5): 589–593. doi:10.1007/BF01161081. S2CID 95449522.
- ^ a b Metcalf Deceased, Robert L.; Horowitz, Abraham Rami (2014). "Insect Control, 2. Individual Insecticides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.s14_s01.
- ^ Herrick, Glenn Washington (1910). "The Snow-white Linden Moth".
- ^ Historic Arsenical Pesticide Research (PDF) (Report). US Environmental Protection Agency Office of Pesticide Programs. 2004. Retrieved 22 January 2020.
EPA banned use of lead arsenate on food crops in 1988.
- ^ Inorganic Arsenicals; Intent to Cancel Registrations for Pesticide Products Registered for Non-Wood Preservative Use; Conclusion of Special Review. Federal Register: 53: 126 (Report). United States Environmental Protection Agency (USEPA). June 1988. p. 24787-24796.
- ^ Shavit, Elinoar; Shavit, Efrat (Spring 2010). "Lead and Arsenic in Morchella esculenta Fruitbodies Collected in Lead Arsenate Contaminated Apple Orchards in the Northeastern United States: A Preliminary Study" (PDF). Fungi Magazine. 3 (2): 11–18.
- ^ Peryea, F. J.; Creger, T. L. (1994-12-01). "Vertical distribution of lead and arsenic in soils contaminated with lead arsenate pesticide residues". Water, Air, and Soil Pollution. 78 (3): 297–306. doi:10.1007/BF00483038. ISSN 1573-2932.