 | |
 | |
| Names | |
|---|---|
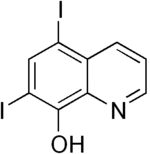
| IUPAC name 5,7-diiodoquinolin-8-ol | |
| Other names Diquinol, iodoxin, diiodoquin, amebaquin | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.362 |
| KEGG | |
| MeSH | Iodoquinol |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C9H5I2NO |
| Molar mass | 396.951 |
| Pharmacology | |
| G01AC01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
The quinoline derivative diiodohydroxyquinoline (INN) or iodoquinol (USAN), can be used in the treatment of amoebiasis.[1]
It is poorly absorbed from the gastrointestinal tract and is used as a luminal amebicide. It acts by chelation of ferrous ions essential for metabolism.[2]
It was discovered by Adco Co. and introduced as diiodohydroxyquinoline.[3]
Susceptibility of Dientamoeba fragilis has been measured.[4]
Iodoquinol is an amebocide used against Entamoeba histolytica, and it is active against both cyst and trophozoites that are localized in the lumen of the intestine. It is considered the drug of choice for treating asymptomatic or moderate forms of amebiasis. The full mechanism of action is unknown. Iodoquinol is used for diseases caused by moderate intestinal amebiasis.
Diodoquin enhances zinc absorption in the zinc deficiency disorder Acrodermatitis enteropathica, probably because Diodoquin act as a zinc ionophore.[5]
See also
References
- ^ Ghaskadbi S, Vaidya VG (March 1989). "In vivo antimutagenic effect of ascorbic acid against mutagenicity of the common antiamebic drug diiodohydroxyquinoline". Mutat. Res. 222 (3): 219–22. doi:10.1016/0165-1218(89)90137-7. PMID 2493578.
- ^ Nagata, Noriyuki; Marriott, Deborah; Harkness, John; Ellis, John T.; Stark, Damien (2012). "Current treatment options for Dientamoeba fragilis infections". International Journal for Parasitology: Drugs and Drug Resistance. 2: 204–215. doi:10.1016/j.ijpddr.2012.08.002. ISSN 2211-3207. PMC 3862407. PMID 24533282.
- ^ Publishing, William Andrew (2013-01-15). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier Science. p. 1312. ISBN 9780080947266.
- ^ Chan FT, Guan MX, Mackenzie AM, Diaz-Mitoma F (May 1994). "Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole". Antimicrob. Agents Chemother. 38 (5): 1157–60. doi:10.1128/aac.38.5.1157. PMC 188168. PMID 8067755.
- ^ Aggett, P.J.; Delves, H.T.; Harries, J.T.; Bangham, A.D. (March 1979). "The possible role of Diodoquin as a zinc ionophore in the treatment of acrodermatitis enteropathica". Biochemical and Biophysical Research Communications. 87 (2): 513–517. doi:10.1016/0006-291X(79)91825-4. PMID 375935.