 | |
 | |
| Names | |
|---|---|
| IUPAC name (E)-octadec-9-enoic acid | |
| Other names (E)-9-octadecenoic acid (9E)-octadecenoic acid 18:1 trans-9 C18:1 trans-9 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.642 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C 18H 34O 2 |
| Molar mass | 282.46 g/mol |
| Appearance | colorless waxy solid |
| Density | 0.8734 g/cm3 |
| Melting point | 45 °C (113 °F) |
| -204.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
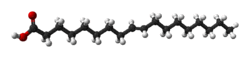
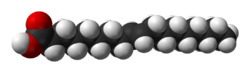
Elaidic acid is a chemical compound with the formula C
18H
34O
2, specifically the fatty acid with structural formula HO(O=)C–(CH2–)7CH=CH–(CH2–)8H, with the double bond (between carbon atoms 9 and 10) in trans configuration. It is a colorless oily solid. Its salts and esters are called elaidates.
Elaidic acid is an unsaturated trans fatty acid, with code C18:1 trans-9. This compound has attracted attention because it is a major trans fat found in hydrogenated vegetable oils, and trans fats have been implicated in heart disease.[1]
It is the trans isomer of oleic acid. The name of the elaidinization reaction comes from elaidic acid.
Occurrence and bioactivity
Elaidic acid occurs naturally in small amounts in caprine and bovine milk (very roughly 0.1% of the fatty acids)[2] and in some meats.[3] It also comprises 2.50% of the fats from the fruit of the durian species Durio graveolens.[4]
Elaidic acid increases plasma cholesterylester transfer protein (CETP) activity which lowers HDL cholesterol.[5]
See also
References
- ^ Tardy, Anne-Laure; Morio, Béatrice; Chardigny, Jean-Michel; Malpuech-Brugère, Corinne (2011). "Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases". Nutrition Research Reviews. 24 (1): 111–7. doi:10.1017/S0954422411000011. PMID 21320382.
- ^ Alonso L, Fontecha J, Lozada L, Fraga MJ, Juárez M (1999). "Fatty acid composition of caprine milk: major, branched-chain, and trans fatty acids". J. Dairy Sci. 82 (5): 878–84. doi:10.3168/jds.S0022-0302(99)75306-3. hdl:10261/113439. PMID 10342226.
- ^ William Stillwell (2016). Membranes and Human Health. An Introduction to Biological Membranes (Second ed.).
- ^ Nasaruddin, Mohd hanif; Noor, Noor Qhairul Izzreen Mohd; Mamat, Hasmadi (2013). "Komposisi Proksimat dan Komponen Asid Lemak Durian Kuning (Durio graveolens) Sabah" [Proximate and Fatty Acid Composition of Sabah Yellow Durian (Durio graveolens)] (PDF). Sains Malaysiana (in Malay). 42 (9): 1283–1288. ISSN 0126-6039. OCLC 857479186. Retrieved 28 November 2017.
- ^ Abbey M, Nestel PJ (1994). "Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet". Atherosclerosis. 106 (1): 99–107. doi:10.1016/0021-9150(94)90086-8. PMID 8018112.