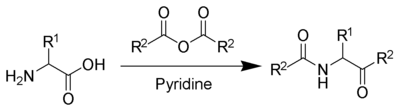
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine.[1][2][3][4][5] It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). In 2016 Schreiner and coworkers reported the first asymmetric variant of this reaction.[6]
With pyridine as a base and solvent, refluxing conditions are required.[7] However, with the addition of 4-dimethylaminopyridine (DMAP) as a catalyst, the reaction can take place at room temperature.[8]
With some acids, this reaction can take place even in the absence of an α-amino group.
This reaction should not be confused with the Dakin reaction.
Reaction mechanism
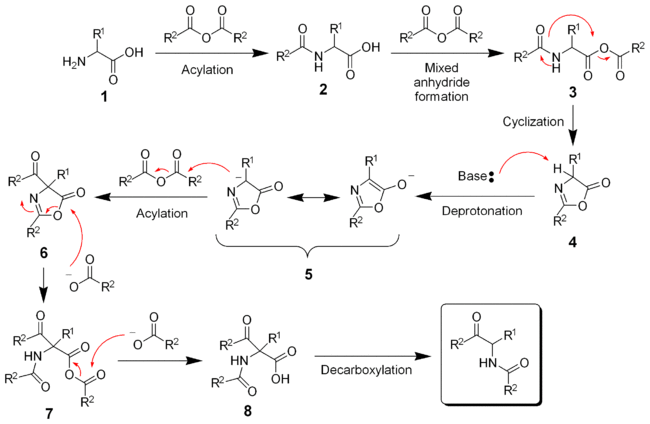
The reaction mechanism involves the acylation and activation of the acid 1 to the mixed anhydride 3. The amide will serve as a nucleophile for the cyclization forming the azlactone 4. Deprotonation and acylation of the azlactone forms the key carbon-carbon bond. Subsequent ring-opening of 6 and decarboxylation give the final keto-amide product.[9][10]
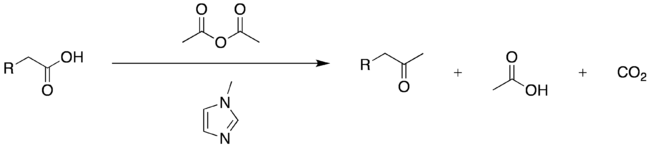
General ketone synthesis
Modern variations on the Dakin–West reaction permit many enolizable carboxylic acids – not merely amino acids – to be converted to their corresponding methyl ketones. For example, β-aryl carboxylic acids can be efficiently converted to β-aryl ketones by treatment of an acetic anhydride solution of the acid with catalytic N-methylimidazole. This reactivity is attributed in part to generation of acetylimidazolium, a powerful cationic acetylating agent, in situ.[11]
See also
- Robinson–Gabriel synthesis - A process for converting the keto-amide products of this reaction into oxazoles
References
- ^ Dakin, Henry Drysdale; West, Randolph (1928). "A General Reaction of Amino Acids". The Journal of Biological Chemistry. 78 (1): 91–104.
- ^ Dakin, Henry Drysdale; West, Randolph (1928). "A General Reaction of Amino Acids. II". The Journal of Biological Chemistry. 78 (3): 745–756.
- ^ Dakin, Henry Drysdale; West, Randolph (1928). "Some Aromatic Derivatives of Substituted Acetylaminoacetones". The Journal of Biological Chemistry. 78 (3): 757–764.
- ^ Wiley, Richard H. (1947). "The Conversion of Amino Acids to Oxazoles". The Journal of Organic Chemistry. 12 (1): 43–46. doi:10.1021/jo01165a006. PMID 20280736.
- ^ Buchanan, G. L. (1988). "The Dakin–West reaction". Chemical Society Reviews. 17: 91–109. doi:10.1039/CS9881700091.
- ^ Wende, Raffael C.; Seitz, Alexander; Niedek, Dominik; Schuler, Sören M. M.; Hofmann, Christine; Becker, Jonathan; Schreiner, Peter R. (2016). "The Enantioselective Dakin-West Reaction". Angewandte Chemie International Edition. 55 (8): 2719–2723. doi:10.1002/anie.201509863. PMID 26804727.
- ^ Wiley, Richard H.; Borum, O. H. (1953). "3-Acetamido-2-butanone". Organic Syntheses. 33: 1. doi:10.15227/orgsyn.033.0001.
- ^ Höfle, Gerhard; Steglich, Wolfgang; Vorbrüggen, Helmut (1978). "4-Dialkylaminopyridines as Highly Active Acylation Catalysts. [New synthetic method (25)]". Angewandte Chemie International Edition in English. 17 (8): 569–583. doi:10.1002/anie.197805691.
- ^ Knorr, Rudolf; Huisgen, Rolf (1970). "Zum Mechanismus der Dakin-West-Reaktion, I Die Reaktion von N-Acyl-sek.-aminosäuren mit Acetanhydrid". Chemische Berichte. 103 (8): 2598–2610. doi:10.1002/cber.19701030831. PMID 5448834.
- ^ Allinger, Norman L.; Wang, Grace L.; Dewhurst, Brian B. (1974). "Kinetic and mechanistic studies of the Dakin-West reaction". The Journal of Organic Chemistry. 39 (12): 1730–1735. doi:10.1021/jo00925a029.
- ^ Tran, Khanh-Van; Bickar, David (2006). "Dakin−West Synthesis of β-Aryl Ketones". The Journal of Organic Chemistry. 71 (17): 6640–6643. doi:10.1021/jo0607966. PMID 16901161.