 | |
| Names | |
|---|---|
| Other names Cobalt trifluoride Cobaltic fluoride Cobalt fluoride Cobaltic trifluoride | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.045 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| CoF 3 | |
| Molar mass | 115.928 g/mol |
| Appearance | brown powder |
| Density | 3.88 g/cm3 |
| Melting point | 927 °C (1,701 °F; 1,200 K) |
| reacts | |
| +1900.0·10−6 cm3/mol | |
| Structure | |
| hexagonal | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions | cobalt(III) oxide, cobalt(III) chloride |
Other cations | iron(III) fluoride, rhodium(III) fluoride |
Related compounds | cobalt(II) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Cobalt(III) fluoride is the inorganic compound with the formula CoF
3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.[1]
The related cobalt(III) chloride is also known but is extremely unstable.[2] Cobalt(III) bromide and cobalt(III) iodide have not been synthesized.
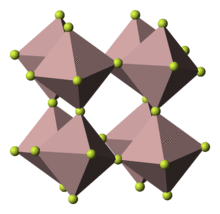
Structure
Anhydrous
Anhydrous cobalt trifluoride crystallizes in the rhombohedral group, specifically according to the aluminium trifluoride motif, with a = 527.9 pm, α = 56.97°. Each cobalt atom is bound to six fluorine atoms in octahedral geometry, with Co–F distances of 189 pm. Each fluoride is a doubly bridging ligand.[3]
Hydrates
A hydrate CoF
3·3.5H2O is known. It is conjectured to be better described as [CoF
3(H
2O)
3]·0.5H2O.[3]
There is a report of an hydrate CoF
3·3.5H2O, isomorphic to AlF
3·3H2O.[3]
Preparation
Cobalt trifluoride can be prepared in the laboratory by treating CoCl
2 with fluorine at 250 °C:[4][3]
- CoCl
2 + 3/2 F
2 → CoF
3 + Cl
2
In this redox reaction, Co2+ and Cl− are oxidized to Co3+ and Cl
2, respectively, while F
2 is reduced to F−. Cobalt(II) oxide (CoO) and cobalt(II) fluoride (CoF
2) can also be converted to cobalt(III) fluoride using fluorine.[3]
The compound can also be formed by treating CoCl
2 with chlorine trifluoride ClF
3 or bromine trifluoride BrF
3.[3]
Reactions
CoF
3 decomposes upon contact with water to give oxygen:
- 4 CoF
3 + 2 H2O → 4 HF + 4 CoF
2 + O2
It reacts with fluoride salts to give the anion [CoF6]3−, which is also features high-spin, octahedral cobalt(III) center.
Applications
CoF
3 is a powerful fluorinating agent. Used as slurry, CoF
3 converts hydrocarbons to the perfluorocarbons:
- 2 CoF
3 + R-H → 2 CoF
2 + R-F + HF
CoF
2 is the byproduct.
Such reactions are sometimes accompanied by rearrangements or other reactions.[1] The related reagent KCoF4 is more selective.[5]
Gaseous CoF
3
In the gas phase, CoF
3 is calculated to be planar in its ground state, and has a 3-fold rotation axis (symmetry notation D3h). The Co3+ ion has a ground state of 3d6 5D. The fluoride ligands split this state into, in energy order, 5A', 5E", and 5E' states. The first energy difference is small and the 5E" state is subject to the Jahn-Teller Effect, so this effect needs to be considered to be sure of the ground state. The energy lowering is small and does not change the energy order.[6] This calculation was the first treatment of the Jahn-Teller Effect using calculated energy surfaces.
References
- ^ a b Coe, P. L. (2004). "Cobalt(III) Fluoride". Encyclopedia of Reagents for Organic Synthesis. J. Wiley. doi:10.1002/047084289X.rc185. ISBN 0471936235.
- ^ Arthur W. Chester, El-Ahmadi Heiba, Ralph M. Dessau, and William J. Koehl Jr. (1969): "The interaction of cobalt(III) with chloride ion in acetic acid". Inorganic and Nuclear Chemistry Letters, volume 5, issue 4, pages 277-283. doi:10.1016/0020-1650(69)80198-4
- ^ a b c d e f W. Levason and C. A. McAuliffe (1974): "Higher oxidation state chemistry of iron, cobalt, and nickel". Coordination Chemistry Reviews, volume 12, issue 2, pages 151-184. doi:10.1016/S0010-8545(00)82026-3
- ^ H. F. Priest (1950): "Anhydrous Metal Fluorides". In Inorganic Syntheses, McGraw-Hill, volume 3, pages 171-183. doi:10.1002/9780470132340.ch47
- ^ Coe, P. L. "Potassium Tetrafluorocobaltate(III)" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rp251.
- ^ Yates, J. H.; Pitzer, R. M. (1979). "Molecular and Electronic Structure of Transition Metal Trifluorides". J. Chem. Phys. 70 (9): 4049–4055. doi:10.1063/1.438027.
