| | |||
| Names | |||
|---|---|---|---|
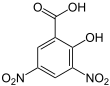
| Preferred IUPAC name 2-Hydroxy-3,5-dinitrobenzoic acid | |||
| Other names 3,5-Dinitrosalicylic acid | |||
| Identifiers | |||
3D model (JSmol) | |||
| 2220661 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.009.278 | ||
| EC Number |
| ||
Gmelin Reference | 5309 | ||
| KEGG | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
| C7H4N2O7 | |||
| Molar mass | 228.116 g·mol−1 | ||
| Appearance | Yellow needles or plates | ||
| Melting point | 182 °C (360 °F; 455 K) | ||
| Soluble | |||
| Solubility in organic solvents | Soluble in ethanol, diethyl ether, benzene | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements | H302, H315, H318, H319, H335 | ||
GHS precautionary statements | P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
3,5-Dinitrosalicylic acid (DNS or DNSA, IUPAC name 2-hydroxy-3,5-dinitrobenzoic acid) is an aromatic compound that reacts with reducing sugars and other reducing molecules to form 3-amino-5-nitrosalicylic acid, which strongly absorbs light at 540 nm. It was first introduced as a method to detect reducing substances in urine by James B. Sumner [2] and has since been widely used, for example, for quantifying carbohydrate levels in blood.[3] It is mainly used in assay of alpha-amylase. However, enzymatic methods are usually preferred due to DNS lack of specificity.[4]
Synthesis
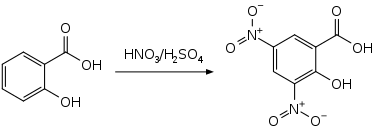
3,5-Dinitrosalicylic acid can be prepared by the nitration of salicylic acid.[5]
References
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–318. ISBN 978-0-8493-0594-8.
- ^ Sumner, J.B. Dinitrosalicylic acid: a reagent for the estimation of sugar in normal and diabetic urine. Journal of Biological Chemistry 47, 5, 1921.
- ^ Description of lab use from the Department of Chemical Engineering, University of Maryland
- ^ Miller, Gail Lorenz (1959). "Use of dinitrosalicylic acid reagent for determination of reducing sugar". Anal. Chem. 31 (3): 426–428. doi:10.1021/ac60147a030.
- ^ Thiel, W.; Mayer, R.; Jauer, E.-A.; Modrow, H.; Dost, H.: Synthesis and Spectral Characterization of Blue Dyes of the Benzene Series in J. Prakt. Chem. (Leipzig) 328 (1986) 497-514, doi:10.1002/prac.19863280406.