 | |
 | |
| Names | |
|---|---|
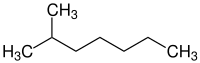
| IUPAC name 2-Methylheptane[1] | |
| Identifiers | |
3D model (JSmol) | |
| 1696862 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.863 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 1262 |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Odourless |
| Density | 698 mg mL−1 |
| Melting point | −112 to −108 °C; −170 to −163 °F; 161 to 165 K |
| Boiling point | 116.8 to 118.4 °C; 242.2 to 245.0 °F; 389.9 to 391.5 K |
| Vapor pressure | 5.3 kPa (at 37.7 °C) |
Henry's law constant (kH) | 2.7 nmol Pa−1 kg−1 |
Refractive index (nD) | 1.395–1.396 |
| Thermochemistry | |
Heat capacity (C) | 252.00 J K−1 mol−1 |
Std molar entropy (S | 356.39 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) | −256.5–−253.9 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) | −5466.7–−5464.3 kJ mol−1 |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements | H225, H304, H315, H336, H410 |
GHS precautionary statements | P210, P261, P273, P301+310, P331 |
| NFPA 704 (fire diamond) | |
| Flash point | 4.4 °C (39.9 °F; 277.5 K) |
| Explosive limits | 0.98–?% |
| Related compounds | |
Related alkanes |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2-Methylheptane is a branched alkane isomeric to octane. Its structural formula is (CH3)2CH(CH2)4CH3.
References
- ^ "2-METHYLHEPTANE - Compound Summary". PubChem Compound. National Center for Biotechnology Information. 26 March 2005. Retrieved 6 March 2012.
