 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2,6-Di-tert-butylphenol | |
| Other names 2,6-Bis(1,1-dimethylethyl)phenol Dibutylphenol 2,6-Bis(tert-butyl)phenol 2,6-Di(1,1-dimethylethyl)phenol 2,6-DTBP Ethanox 701 Ethyl 701 Ethyl AN 701 Irganox L 140 Isonox 103 TK 12891 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.441 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2430, 3077 |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C14H22O | |
| Molar mass | 206.329 g·mol−1 |
| Appearance | Low-melting colourless solid |
| Melting point | 34 to 37 °C (93 to 99 °F; 307 to 310 K) |
| Boiling point | 253 °C (487 °F; 526 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements | H315, H319, H400, H410 |
GHS precautionary statements | P264, P273, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362, P391, P501 |
| Flash point | 118°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
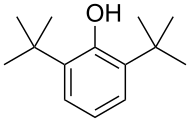
2,6-Di-tert-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics.[1] Illustrative of its usefulness, it prevents gumming in aviation fuels.
Production
2,6-Di-tert-butylphenol is prepared industrially via the Friedel–Crafts alkylation of phenol with isobutene catalyzed by aluminium phenoxide:
- C6H5OH + 2 CH2=C(CH3)2 → ((CH3)3C)2C6H3OH
In this way, approximately 2.5M kg/y are produced.[2]
Applications
Its dominant use is as an antioxidant.
2,6-di-tert-butylphenol is a precursor to more complex compounds used as antioxidants and light-protection agents for the stabilization for polymers. Of particular note is methyl-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate (CAS# 6386-38-5), which is formed by the Michael addition of methyl methacrylate. This compound is used as a feedstock in the synthesis of more complex antioxidants such as Irganox 1098. 2,6-Di-tert-butylphenol is also used in the synthesis of CGP-7930, probucol, and nicanartine.
Safety and regulation
The LD50 is 9200 mg/kg, indicating a low toxicity.[2]
2,6-Di-tert-butylphenol is covered by the U.S. Department of Transportation Code of Federal Regulations 49 CFR 172.101, Appendix B (20 Dec 2004). This substance is designated by the U.S. Department of Transportation (DOT) as a marine pollutant.
See also
References
- ^ Peter P. Klemchuk (2005). "Antioxidants". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_091.
- ^ a b Helmut Fiege; Heinz-Werner Voges; Toshikazu Hamamoto; Sumio Umemura; Tadao Iwata; Hisaya Miki; Yasuhiro Fujita; Hans-Josef Buysch; Dorothea Garbe (2002). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.