 | |||
| | |||
| Names | |||
|---|---|---|---|
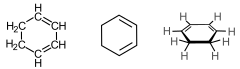
| IUPAC name Cyclohexa-1,3-diene | |||
| Other names 1,3-Cyclohexadiene, 1,2-Dihydrobenzene, 1,3-CHD | |||
| Identifiers | |||
3D model (JSmol) | |||
| 506024 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.878 | ||
| EC Number |
| ||
Gmelin Reference | 1657 | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
| C6H8 | |||
| Molar mass | 80.13 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.841 g/cm3 | ||
| Melting point | −98 °C (−144 °F; 175 K) | ||
| Boiling point | 80 °C (176 °F; 353 K) | ||
| -48.6·10−6 cm3/mol | |||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements | H225, H335 | ||
GHS precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+361+353, P304+340, P312, P370+378, P403+233, P403+235, P405, P501 | ||
| Flash point | 26 °C (79 °F; 299 K) c.c. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Cyclohexa-1,3-diene is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of 1,3-cyclohexadiene is terpinene, a component of pine oil.
Synthesis
Cyclohexadiene is prepared by the dehydrobromination of 1,2-dibromocyclohexane:[1]
- (CH2)4(CHBr)2 + 2 NaH → (CH2)2(CH)4 + 2 NaBr + 2 H2
Reactions
Useful reactions of this diene are cycloadditions, such as the Diels-Alder reaction.[2]
Conversion of cyclohexa-1,3-diene to benzene + hydrogen is exothermic by about 25 kJ/mol in the gas phase.[3][4]
- cyclohexane → cyclohexa-1,3-diene + 2 H2 (ΔH = +231.5 kJ/mol; endothermic)
- cyclohexane → benzene + 3 H2 (ΔH = +205 kJ/mol; endothermic)
- cyclohexa-1,3-diene → benzene + H2 (ΔH = -26.5 kJ/mol; exothermic)
Compared with its isomer cyclohexa-1,4-diene, cyclohexa-1,3-diene is about 1.6 kJ/mol more stable.[5]
Cyclohexadiene and its derivatives form metal-alkene complexes. Illustrative is [C6H8)Fe(CO)3], an orange liquid. This complex reacts with hydride-abstracting reagents to give the cyclohexadienyl derivative [C6H7)Fe(CO)3]+.[6] Cyclohexadienes react with ruthenium trichloride to give (Benzene)ruthenium dichloride dimer.[7]
See also
References
- ^ Schaefer, John P.; Endres, Leland (1967). "1,3-Cyclohexadiene". Organic Syntheses. 47: 31. doi:10.15227/orgsyn.047.0031.
- ^ Sanjeeva Rao Guppi, George A. O'Doherty, "1,3-Cyclohexadiene" Encyclopedia of Reagents for Organic Synthesis, 2008 John Wiley & Sons. doi:10.1002/047084289X.rn00921
- ^ US National Institute of Standards and Technology, NIST Chemistry WebBook 1,3-Cyclohexadiene Benzene
- ^ J. Sherman The heats of hydrogenation of unsaturated hydrocarbons Archived 2011-07-14 at the Wayback Machine Journal of the American Oil Chemists' Society; Volume 16, Number 2 / February, 1939
- ^ NIST Chemistry WebBook 1,4-Cyclohexadiene
- ^ Pearson, Anthony J.; Sun, Huikai (2008). "Cyclohexadieneiron Tricarbonyl". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00791.
- ^ Bennett, M. A.; Huang, T. N.; Matheson, T. W.; Smith, A. K. (1982). "(η6-Hexamethylbenzene)ruthenium Complexes". Inorganic Syntheses. 21: 74–78. doi:10.1002/9780470132524.ch16.