| | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name 1,2-Dichloroethene | |||
| Other names 1,2-Dichloroethylene 1,2-DCE sym-Dichloroethylene | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.956 | ||
| KEGG | |||
PubChem CID | |||
| UNII |
| ||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
| C2H2Cl2 | |||
| Molar mass | 96.95 g/mol | ||
| Appearance | clear liquid[1] | ||
| Odor | acrid, chloroform-like[1] | ||
| Density | Z: 1.28 g/cm3 E: 1.26 g/cm3 | ||
| Melting point | Z: −81.47 °C E: −49.44 °C | ||
| Boiling point | Z: 60.2 °C E: 48.5 °C | ||
| |||
| Z: 1.9 D E: 0 D | |||
| Hazards | |||
| Flash point | 2–4 °C; 36–39 °F; 275–277 K | ||
| Explosive limits | 5.6–12.8%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 770 mg/kg (oral, rat) 1275 mg/kg (oral, rat, trans-isomer)[2] | ||
LC50 (median concentration) | 21,273 ppm (mouse, 6 hr, trans-isomer)[2] | ||
LCLo (lowest published) | 16,000 ppm (rat, 6 hr, cis-isomer)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 200 ppm (790 mg/m3)[1] | ||
REL (Recommended) | TWA 200 ppm (790 mg/m3)[1] | ||
IDLH (Immediate danger) | 1000 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
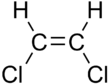
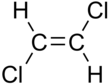
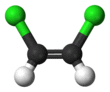
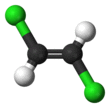
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a highly flammable, colorless liquid with a sharp, harsh odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have few industrial applications,[3] although they are fundamental given their simple stoichiometries.
Production
cis-DCE, the Z isomer, is obtainable by the controlled chlorination of acetylene:
- C2H2 + Cl2 → C2H2Cl2
Industrially both isomers arise as byproduct of the production of vinyl chloride, which is produced on a vast scale. Unlike vinyl chloride, the 1,2-dichloroethylene isomers do not polymerize.[3]
trans-DCE has applications including electronics cleaning, precision cleaning, and certain metal cleaning applications.[4]
E-Z relative stability
In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.[5]
Safety
These compounds have "moderate oral toxicity to rats".[3]
See also
- 1,1-Dichloroethene
- 1,2-Dichloroethane, which is also often abbreviated as 1,2-DCA
References
- ^ a b c d e f g NIOSH Pocket Guide to Chemical Hazards. "#0195". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c "1,2-Dichloroethylene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c E.-L. Dreher; T. R. Torkelson; K. K. Beutel (2011). "Chlorethanes and Chloroethylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01. ISBN 978-3527306732.
- ^ "Chlorinated Solvents and Feed Stock - Axiall". Archived from the original on 2016-04-08. Retrieved 2016-03-23.
- ^ Pitzer, Kenneth S.; Hollenberg, J. L. (1954). "cis- and trans-Dichloroethylenes. The Infrared Spectra from 130–400 Cm.–1 and the Thermodynamic Properties". J. Am. Chem. Soc. 76 (6): 1493–1496. doi:10.1021/ja01635a010.
External links
- International Chemical Safety Card 0436
- NIOSH Pocket Guide to Chemical Hazards. "#0195". National Institute for Occupational Safety and Health (NIOSH).